


| Item | Index |
|---|---|
| Appearance | White powder |
| TiO2 content | ≥98.0% |
| Moisture content at 105℃ | ≤0.4 |
| PH | 6.5-8.5 |
| Residue on sieve of 45μm | ≤0.03% |
| Color(L*) | ≥96.0% |
| Oil absorption, g/100g | ≤23 g/100g |
| Resistivity | ≥30Ω·m |
| Inorganic coating | Alumina, Phosphate |
| Organic treatment | Not present |
| Key features: |
1)Excellent whiteness and glossiness 2)Favorable covering power and lightening power 3)Good dispersion, low oil absorption |
| Recommended applications: | 1)Interior wall coating 2)interior plastic pipe, film, rubber, leather, paper, and other fields. |
| Packing | In 25KG /500KG /1000KG bag or as per customer’s requirement |
|---|---|
| Storage | This product should not be stored outside or exposed to temperature extremes or to moisture.
To ensure optimum performance, it is recommended that the product is used on a first in, first out basis from receipt of shipment. |
Tel: 0086-25-52397808
E-mail: info@titanium-dioxide.net


| Common Names | TITANIUM DIOXIDE | ||
|---|---|---|---|
| Structure |
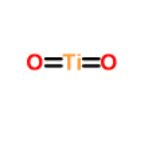
|
||
| CAS No. | 13463-67-7 | Boiling Point (℃) | 2900 |
| Molecular Weight | 79.866 | Melting Point (℃) | 1840 |
| Appearance | white powder | Bulk density | 3.84 |
| HS Code | 3206111000 | Flash Point (℃) | 2500-3000 |
| Safety Phrases | S2;S25;S26;S36;S36/S37 | ||
|---|---|---|---|
| RIDADR | No hazardous good according to the regulation. | ||
| WGK Germany | NONE | ||
| Packaging Group | NONE | ||
| Hazard Class | NONE | ||
| SYMPTOMS | PREVENTION | FIRST AID | |
| Inhalation | Cough. Sore throat. Redness. Burning sensation. Itching. | Use local exhaust or breathing protection. | Fresh air, rest. |
| Eyes | Redness. Pain. | Protective gloves. | Rinse opened eye for several minutes under running water. Then consult a doctor. |
| Ingestion | Abdominal pain. Nausea. Vomiting. | Do not eat, drink, or smoke during work. Wash hands before eating. | Rinse mouth. Induce vomiting (ONLY IN CONSCIOUS PERSONS!). Refer for medical attention . |
Production process of titanium dioxide
The production of titanium dioxide typically involves two main processes: the chloride process and the sulfate process. The sulfate process is the most commonly used method for producing titanium dioxide. In the sulfate process, titanium dioxide is derived from ilmenite ore or rutile, which are natural sources of titanium dioxide. The ore is first processed to extract titanium dioxide by reacting it with sulfuric acid. This process yields a titanium dioxide slurry, which is further processed to obtain the final titanium dioxide pigment.